- Research
- Open access
- Published:
Breastfeeding, nutrition and type 1 diabetes: a case-control study in Izmir, Turkey
International Breastfeeding Journal volume 17, Article number: 42 (2022)
Abstract
Background
The relationship between infant breastfeeding and type 1 diabetes mellitus (DM) is unclear but it has been suggested that there may be a link between many environmental factors, including dietary antigens affecting diabetes epidemiology.
The main objective of this study is to investigate nutritional risk factors, especially breastfeeding early in life that may be associated with the development of type 1 DM and to determine the relationship these factors have with the disease.
Methods
This research is a case-control study and was carried out in Ege University Children’s Hospital in İzmir, Turkey between 13 January 2020 and 5 March 2020. A total of 246 children aged between 4 and 14 years were included in the study. The case group consisted of patients diagnosed with type 1 DM followed-up by Ege University Children’s Hospital’s Endocrinology Unit and the control group included non-diabetic children attending the same hospital’s General Pediatric Outpatient Clinic. A structured questionnaire was created by the researchers after reviewing the literature related to nutritional and other risk factors for type 1 DM. The questionnaire was administered by interviewing the parents and it was related to the child, mother and family of the child. In this study, breastfeeding duration was defined as the total duration of breastfeeding and exclusive breastfeeding meant that the child received only breast milk from the mother.
Results
The mean age at diagnosis was 6.30 ± 4.03 years for cases and 7.48 ± 2.56 years for controls. We found that each monthly increase in exclusive breastfeeding duration provided a 0.83-fold (95% CI 0.72, 0.96) decrease in the risk of type 1 DM. Introduction of cereals in the diet at the sixth month or earlier was associated with a 2.58-fold (95% CI 1.29, 5.16) increased risk.
Conclusions
Determining the contribution of exclusive breastfeeding to the disease is important in establishing preventive policies. A longer duration of exclusive breastfeeding may be an important role in preventing the disease. This free intervention that truly works will be cost-effective. Future studies are needed to clarify the role of both exclusive and non-exclusive breastfeeding on the development of type 1 DM.
Background
Diabetes mellitus (DM) is a chronic metabolic disease characterized by hyperglycemia due to impairments in either insulin secretion and / or insulin effect [1]. As of today, 537 million people worldwide have diabetes [2]. This number is estimated to reach 643 million in 2030 and 783 million in 2045, which can be considered alarming levels [2].
Type 1 DM is characterized by insulin deficiency and hyperglycemia, usually starting in childhood, when the beta-cells of the pancreas are destroyed by autoimmune or non-autoimmune processes [2]. In individuals with genetic predisposition (human leukocyte antigen or HLA groups at risk), autoimmunity is triggered by the effect of environmental factors (viruses, toxins, emotional stress, others) and progressive beta-cell damage begins. Clinical symptoms of diabetes occur when beta-cell reserves are reduced by 80–90% [3].
It has been suggested that there are many environmental factors, including dietary antigens [4,5,6], as well as genetic risk factors [7,8,9,10,11] that affect the epidemiology of type 1 DM [12]. Although not all genotypes with risk have yet been identified, only about 10–15% of individuals at genetic risk develop type 1 DM [5]. In studies conducted on migrants, it has been shown that the incidence of type 1 DM increases in those who migrate from a region where the incidence of type 1 DM is low to a region with high incidence, and the effect of environmental conditions has been emphasized [13]. These data were found to be consistent with the results of studies finding that environmental triggers increase and accelerate the development of clinical type 1 DM despite lower genetic predisposition [13].
Some nutritional factors contribute to the development of the disease. Studies in 40 countries worldwide have shown that dietary patterns may impact the development of type 1 DM [14]. Vitamin D, another nutritional factor, may have a protective effect on glycemic control in patients with type 1 DM [15] and according to a birth cohort study, the provision of vitamin D supplementation for infants early in life could help to reduce the risk of the disease [16]. The introduction of cow’s milk-based infant formulas in the first three postnatal months was found to be associated with an increase in pancreatic beta-cell auto-antibodies [17]. However, another study had shown that cow’s milk did not play an important role in the development of type 1 DM [18].
Although many studies have been performed to investigate the role of nutrition in pregnancy and early in life on type 1 DM, the results have been inconsistent. Breastfeeding [19], probiotic supplementation [20], vitamin C, and zinc supplementation [21] have been shown as possible protective factors against type 1 DM whereas early exposure to eggs, gluten [22, 23] and vegetables [24] might increase the risk.
Studies with school-age children have shown that diabetic children are significantly more prone to stress and depression compared to non-diabetic children [25]. Beyond the psychological and somatic effects of the disease on the individuals, diabetic individuals also encounter socio-economic consequences affecting their families and entire societies [26]. Frequent co-morbidities further increase negative socioeconomic consequences, especially in low- and middle-income countries [26].
According to the Social Security Institution’s data in Turkey, the costs of diabetes and its complications amount to approximately 23% of the total health expenditure [27]. In addition, indirect costs such as the loss of productivity of diabetics, the persons caring for the patient and their family are not included in these cost estimates. Therefore the cost does not reflect the psychosocial effects of the losses of quality-adjusted life years. Knowledge of modifiable environmental risk factors in type 1 DM can assist authorities in planning and implementing preventive policies to reduce the burden of the disease. It is as yet uncertain how and which nutritional or other environmental factors are important in the development of type 1 DM. Moreover, epigenetic mechanisms are not clearly defined.
The main objective of this study is to investigate potential nutritional risk factors, especially breastfeeding early in life, that may be associated with the development of type 1 DM and to determine the relationship of these factors with the disease, independent of other established risk factors.
Methods
Participants
A case-control study was carried out at Ege University Children’s Hospital, İzmir City, Turkey, over a period of two months from January to March 2020.
A minimum sample size of 105 cases and 105 controls with a total of 210 participants was calculated with G-Power using the t-test group, with an effect size of 0.5, an error margin of 0.05, and a power of 95%. About 20% more sample size was added to account for possible non-response and a total of 246 children (120 cases and 126 controls) were included in the study.
The study data were collected at Ege University Faculty of Medicine Children’s Hospital in Bornova, Izmir between 13 January 2020 and 5 March 2020. Children and their parents who attended the general pediatrics and endocrinology / metabolic diseases outpatient clinics of the hospital and who met the study criteria were examined. The case group consisted of 120 children in the age group of 4–14 years who were diagnosed with type 1 DM based on World Health Organization and International Diabetes Federation guidelines [28] and who were being followed-up at Ege University Children’s Hospital Endocrinology / metabolic diseases outpatient clinic.
The diabetes outpatient clinic is held once a week (on Thursdays) and on the first Monday of every month. The mean number of diabetic patients attending the research was 15 patients per day. The control group comprised 126 non-diabetic children selected from the general pediatric outpatient clinic of the same hospital. A questionnaire was applied face-to-face to the parents of the children. All questions in the study were asked to the parents and separately written informed consent was obtained from children and their parents. In addition, the files of the case group were examined and the date of diagnosis, height, body weight and HbA1c levels at the time of diagnosis were collected as data.
Children who were followed up in the Endocrine and Metabolic Diseases Outpatient Clinic, diagnosed with type 1 DM and aged between 4 and 14 years were included in the case group. Children who attended the General Pediatrics Outpatient Clinic, were not diagnosed with type 1 DM, and aged 4–14 years were included in the control group. Those who did not want to share their information and could not remember answers to the study questions were excluded. The response rates were 96 and 91% among cases and controls, respectively, for all eligible cases and controls attending the hospital.
Questionnaires
A structured questionnaire was created by the researchers after reviewing the literature related to nutritional and other risk factors for type 1 DM [21, 29,30,31,32,33,34]. The questionnaire was administered by interviewing the parents and its content was related to the child, mother and family of the child. For children: anthropometric data, breastfeeding duration, infant formula consumption, the introduction of some foods into the diet, infections, supplementations (vitamin D and probiotic) early in life and physical activity were questioned; for mothers, anthropometric data and history during pregnancy; for family, socio-demographic characteristics such as education, whether the child lived with parents, and family history were asked. In addition, the case group was examined about the age at diagnosis of the disease, the HbA1c level and the percentiles at diagnosis.
In this study, breastfeeding duration was defined as the total duration of breastfeeding and exclusive breastfeeding meant that the child received only breast milk (no other liquids or solids given, not even water with the exception of oral rehydration solution, or drops / syrups of vitamins, minerals or medicines) from the mother [35].
The percentiles were calculated based on the percentile values table of Neyzi et al. [36]. Parents’ body mass index (BMI) was classified according to the World Health Organization’s obesity scale [37]. Finally, high-intensity physical activity was defined as “physical activities that increase the maximum heart rate by 70 − 85%” [38]. Examples of physical activities were given (running, basketball, football, tennis, swimming, skipping rope) by the researcher.
Statistical analysis
The data were analyzed by using SPSS software. The quality of the data had been checked prior to analysis. Descriptive variables of cases and controls were compared with Student t-tests (continuous variables), Mann Whitney U tests (non-parametric) and chi-square tests (categorical variables). In order to reveal the relationship between significant parameters and the development of type 1 DM independently from other factors, age and sex-adjusted logistic regression analysis were performed. Since the difference in mean ages of the two groups was found to be significant (both age of enrolment in the study and age at diagnosis type 1 DM), other variables were evaluated adjusting for age and gender.
General pediatric outpatient clinic admissions are due to newly developing acute conditions and 85–90% are first visits to the hospital. Ten to 15 % are invited for follow-up one month later, so the follow-up is also at the same age. If they also have a chronic condition, they are referred to pediatric specialization clinics and start follow-up in those clinics.
Among the cases, six were diagnosed with type 1 DM at zero years, three of whom were excluded from the multivariate analysis since they were diagnosed in their first month of life, so the diagnosis would be before the environmental exposures could happen. The remaining three children were diagnosed at 10, 11 and 11 months, thus they were kept in the analysis since they could be exposed to potential nutritional risk factors in question.
Sex and age-adjusted multivariable logistic analysis, adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were used to identify possible risk factors of the disease. In all analyses, p < 0.05 was considered statistically significant. The dependent variable was having type 1 DM. Maternal factors, family history, family characteristics, nutritional characteristics early in life were the independent variables.
Population Attributable Risk (PAR) and Population Attributable Risk Percent (PAR %) were calculated to estimate the proportion of cases for whom the disease is attributable to exclusive breastfeeding and to estimate the excess rate of type 1 DM in the study population of both exposed and non-exposed children that is attributable to being non-breastfed exclusively up to the first six months. This measure was calculated as [39]:
Results
Characteristics of children
A total of 246 children were included in the study, with 120 cases and 126 controls. The mean age of the case and control groups was 10.43 ± 3.31 and 7.48 ± 2.56 years, respectively (p < 0.05). The cases’ mean age at diagnosis was 6.30 ± 4.03 years and was found to be significantly lower than the control group. The mean duration of their disease was 4.2 ± 3 .85 years. The mean height percentile was higher in controls (means 45.66 ± 31.16 and 58.00 ± 31.88, p = 0.003) and the mean BMI percentile was higher in the case group (means 55.20 ± 29.86 and 40.32 ± 35.02, p < 0.001). A significant difference was found in the family history of type 1 DM. There was a type 1 DM history in 10.7% of the case group and 0.8% in the control group (p = 0.001). No significant difference was found in the child’s living status with parents and parents’ education level. However, a significant difference was found in physical activity levels (p = 0.014). There was no difference between the duration of vitamin D use. In both groups, no infant was supplemented with probiotics in the first year postpartum. The controls’ rate of living in urban areas was found to be significantly higher (Table 1).
Maternal characteristics
The mean birth interval was higher in the case group. A significant difference was found in the birth intervals with univariate analysis (p = 0.036) but not in multivariate analysis. Those with a birth interval of more than six years constituted 20.7% of the cases and 8.0% of controls (Table 2). In the case group, no mother was supplemented with probiotics during pregnancy and 98.4% of the control group were not supplemented with probiotics during pregnancy.
Nutritional profiles of children
The mean duration of exclusive breastfeeding was higher in the control group (p = 0.009). In the case group, the rate of exclusive breastfeeding for less than one month was 47.8, and 30.6% in the controls (Table 3). This difference was statistically significant (p = 0.037). No statistically significant differences were found between colostrum consumption, total breastfeeding duration, infant formula consumption and formula preferences.
No statistically significant difference was observed between which month the cow’s milk, eggs, fruits, vegetables, and berry fruits were introduced. However the introduction of cereals was statistically significant and the cases’ introduction to them was earlier (p = 0.008). For the case group 5.5% were introduced to cereals before the sixth month as compared to 3.2% of controls, while 44.2% of controls were introduced to cereals after the eighth month, compared to 24.7% of cases (Table 4).
Multivariate analysis
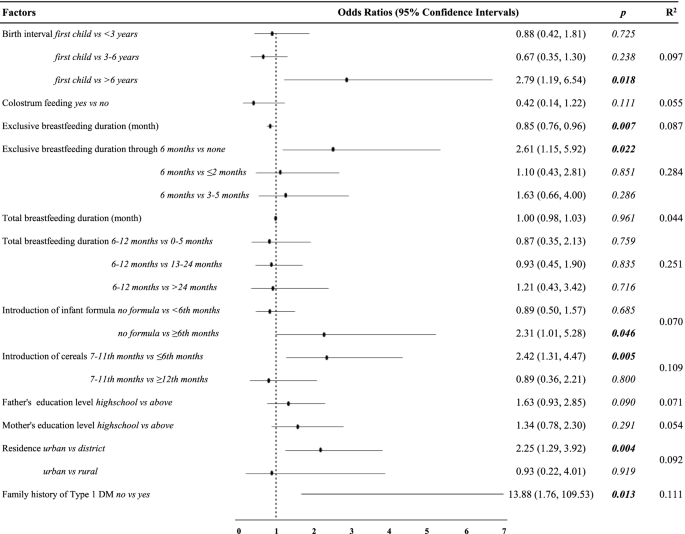
According to non-parametric correlation analyses, exclusive breastfeeding duration and total breastfeeding duration were not found to be associated with age when type 1 DM was diagnosed. The birth interval was found to be significant in the age and sex-adjusted regression analysis. In addition, regardless of age and gender, it was observ ed. that the risk of type 1 DM decreased 0.85 (p = 0.007; 95% CI0.76, 0.96) times with each monthly increase in the duration of exclusive breastfeeding (Fig. 1). Having a birth interval of more than six years increased the risk of the disease by 2.79 (p = 0.018; 95% CI 1.19, 6.54) times.
According to results of the multivariate logistic regression, longer exclusive breastfeeding duration, living in a rural area and not consuming infant formula were identified as protective factors. Although there was no significant difference found in type 1 DM risk with introduction to cereals at 12 months and after, it was found that the introduction to cereals at the sixth month and earliere increased the risk of type 1 DM by 2.58 (p = 0.008; 95% CI 1.29, 5.16) times compared to between months7–11, independent of other risk factors. Similarly, infant formula consumption after the sixth month was associated with an increased risk of type 1 DM compared to no infant formula consumption (Table 5).
Sensitivity analyses
The potential impacts on our results of age at which the cases developed DM, (whether including or excluding data for the three children who were diagnosed at the first month after birth) and with data missing for the father’s education level variable, was assessed using multiple imputations, as described in the Supplementary Data. Among the cases, six of them were diagnosed with type 1 DM in the first year of life, three of whom were excluded from the multivariate analysis since they were diagnosed in their first month of life, so the diagnosis would have occurred before the environmental exposures could happen. The remaining one child was diagnosed at ten months and two children at 11 months, thus they were kept in the analysis since they could have been exposed to the potential nutritional risk factors in question.
The main analyses were repeated after adjusting for age and the father’s education level. Multiple imputations changed some of the conclusions based on the research sample as attached (Supplementary Table 1). The missing data on the father’s education level in the study was assessed using multiple imputations and this did not change the conclusions. So the model excluding the father’s education level and cases that developed type 1 DM before the first month was used.
PAR was calculated as 0.111 in the study and PAR% was calculated as 38.3% .
Discussion
Elimination of preventable environmental risk factors associated with type 1 DM is an important step in the prevention of the disease. However, it has not been precisely explained which factors play a key role and when and in which situations the factors should be eliminated [22]. In this research we have explored possible preventable environmental triggers and determinants, especially breastfeeding early in life.
We found that each monthly increase in the duration of exclusive breastfeeding but not total breastfeeding provides a reduction in type 1 DM risk. However, introducing the cereals before the sixth month was found to be an important risk factor. The birth interval which was significant in univariate analyzes, lost its significance in multivariate analysis.
Breastfeeding
The effect of breast milk, the first food of the newborn, on type 1 DM is a controversial issue. There are many studies in the literature that show no effect [40], a protective effect [19] and an effect [21]. It has been suggested that the protective effect of breastmilk is through reducing neonatal intestinal permeability [41]. The World Health Organization recommends feeding exclusively breast milk in the first six months of life and breastmilk up to the age of two, because feeding children with exclusively breast milk for the first six months after birth prevents diarrhea, respiratory diseases and provides all the nutrients and fluids the infant needs for optimal growth and development [42]. For participants in our study, it was observed that the rates of those who did not receive breast milk at all or those who were exclusively breastfed for less than a month were quite high. It has been observed that the accomplishment rate of the World Health Organization target for six months exclusive breastfeeding is low.
According to the Turkey Demographic and Health Survey data 2018, approximately two in five children were exclusively breastfed up to six months old and the proportion of children who are exclusively breastfed decreases with age; from 59% among 0–1 month-old infants to 14% among 4–5 months old infants [43]. On the other hand, the National Immunization Survey results indicated that only one in four children was breastfed exclusively through six months in the U.S. [44]. In our study, only the median month of breastfeeding was close to the Turkey Demographic and Health Survey data. The exclusive breast milk receiving rates through six months were found to be lower than the worldwide, National Immunization Survey and Turkey Demographic and Health Survey data in both the case and control groups. This was not surprising because our sample was quite low compared to the aforementioned samples, and the data mentioned reflected a population of children younger than two years old in 2018. So the mean age of the children in our study was higher. This result may be different in studies to be conducted with a larger population and adjusted for age. There are large differences in breastfeeding rates between regions, between and within countries. But unfortunately, these rates are insufficient both in the world and in Turkey. We can estimate that 38.3% of type 1 DM cases would be avoided by an increase in the proportion of infants exclusively breastfed to six months. Keep in mind that almost two in five infants who are not breastfed exclusively for the first six months will have type 1 DM so any intervention that can promote breastfeeding may have a big impact in preventing the disease.
In the study of Çarkçı and Altuğ (2020), conducted in the same city as this study (İzmir) the rate of children with type 1 DM who received exclusive breast milk up to the first six months was found to be more than four times compared to our study [45]. While asking the duration of exclusive breastfeeding, the definition of exclusive breastfeeding was explained as “the total time in which the baby takes only breast milk, and no other liquid (including water) or solids other than oral rehydration solution or vitamins, minerals or drugs/syrups are given” in this study. While making a statement, after the parents answered, “Have you ever given water during this period?” was asked again to be sure. In this process, there were parents who changed their answers after the second question. Therefore, different results may have been obtained in studies where this distinction was not made clear.
There are many studies on the relationship between breastfeeding and type 1 DM. Holmberg et al. (2007) found that the duration of total breastfeeding for less than four months is a risk factor for the development of beta-cell autoimmunity in children under five years old. The same study reported that the duration of exclusive breastfeeding for less than four months increased the risk of developing beta-cell autoantibodies two times [17]. In another study, it was shown that the risk of type 1 DM in childhood can be reduced by 15%, even by breastfeeding exclusively in the early weeks of life. However, the observed relationship between exclusive breastfeeding and type 1 DM could not be explained independently of certain risk factors for DM such as gestational DM, birth weight, gestational age, maternal age, birth order and mode of delivery [19]. However in a series of prospective and birth cohort studies investigating the relationship between breastfeeding and the development of islet autoimmunity, no effect of breastfeeding has been reported [46, 47]. Similarly, a series of prospective studies investigating the relationship between breastfeeding and the development of type 1 DM reported that breastfeeding had no effect [48, 49].
We found that longer exclusive breastfeeding duration was a significant protective factor against type 1 DM but the same effect was not observed with total breastfeeding duration. In some studies, exclusive breastfeeding and total breastfeeding duration were not compared and the duration of total or any breastfeeding was researched. Therefore the differences in studies’ results can be attributed to their methods.
In addition to distinguishing between exclusive breastfeeding and total breastfeeding, there are also differences between studies in defining exclusive breastfeeding. For instance, in two Large Scandinavian Birth Cohorts, breastfed infants were found to be at doubled risk of type 1 DM compared to infants who did not receive breast milk at all but no evidence indicated that longer duration of breastfeeding was associated with a reduced risk of the disease [50].
Similarly, in two large cohort studies, breastfeeding duration was not associated with type 1 DM [51, 52]. Infants classified as exclusively breastfed were allowed water-based drinks in the aforementioned study and duration of exclusive breastfeeding was not taken into account.
As can be seen, many studies only look at total breastfeeding duration without making any distinction between exclusive breastfeeding duration and total breastfeeding duration. In addition, striking differences in the breastfeeding practices of governments and health authorities may be a confounding factor in the results of the studies. Positive social norms that support and encourage breastfeeding, including in public spaces, encourage mothers to breastfeed [53]. As observed in our study, the duration of exclusive breastfeeding of children after birth is quite low and it has been observed that parents do not attach sufficient importance to the period of exclusive breastfeeding.
Support from trained counselors and peers, including mothers and other family members, is as important as postpartum health care in maintaining breastfeeding in communities. The support of men, spouses and partners should not be ignored in this process [53]. In studies on breastfeeding, the mother has always been at the center and studies on the role of fathers / partners are insufficient. Tohotoa et al. highlighted the importance of the role of fathers in encouraging and supporting a successful breastfeeding process [54]. Moreover, paternal practical, physical and emotional support could make a difference [54].
When challenges experienced by mothers are shared with their partners, babies might have a better chance of receiving exclusive breast milk for the recommended six months and could keep going on breastfeeding for up to two years. In this way, the early introduction of complementary foods, especially cereals, which we found a significant risk factor for type 1 DM in our study, could be prevented.
Cow’s milk and infant formula consumption
Early exposure to cow’s milk proteins has been studied in terms of beta-cell autoimmunity and the risks of clinical disease development [55]. Early introduction of cow’s milk proteins into the diet may trigger inflammation of the intestinal mucosa and increase intestinal permeability [56]. The introduction of infant formula reflects the total duration of the exclusive breastfeeding [31]. Therefore, it should be considered together with the duration of exclusive breastfeeding. These may have led to contradictory results [31].
Some studies have shown that early exposure to cow’s milk proteins increases the risk of beta-cell autoimmunity [57] and type 1 DM [58] while others found no relationship between type 1 DM and cow’s milk proteins [31, 59]. We also did not find an association with the timing of cow’s milk introduction. It has been observed that consumption of infant formula at six months and later increased the risk of type 1 DM in this research. However, while the risk of type 1 DM was expected to increase with the consumption of infant formula at six months and earlier compared to those who did not consume it, a statistically significant change was not observed.
This result may be explained in three ways: First, there may be a bias in choosing the control group from the same tertiary care and university hospitals with type 1 DM patients. Considering the socioeconomic status of children attending a university hospital, infant formula may have been introduced earlier than the general community and might not represent the healthy control group clearly. Second, there may have been a response bias. Third, since the mean age of children with type 1 DM is significantly higher, parents may have recall bias. Although it is easy to identify potential sources of bias, it is not possible to predict the true impact of these biases on results.
Introduction to cereals
Gluten, a protein found in barley, wheat and rye has been hypothesized to be one of the nutritional risk factors related to the development of type 1 DM [60]. A study in non-obese diabetic rats concluded that the intra-epithelial infiltration of T cells, the incidence of autoimmune type 1 DM and enteropathies decreased with a gluten-free diet compared to the controls [61]. Introduction of gluten before four months of age was associated with an increased risk of type 1 DM in another study [62]. These results were explained by the hypothesis that the gluten-free diet may prevent gliadin peptides from crossing the intestinal barrier by reducing intestinal permeability, thus preventing the development of pancreatic autoimmunity [63]. Our study supports these arguments since introducing cereals before the sixth month was found to be an important risk factor.
However in our study, the cereals were not questioned for their gluten content, they were questioned overall, but wheat production and consumption ranks in the first place among cereals in Turkey [64]. So wheat-containing cereals (including gluten) are expected to be added into the diet of infants in the transition to complementary foods at first. Nevertheless, it could not be confirmed specifically that gluten exposure was earlier in cases, but it was found that cereal introduction prior to the sixth month was associated with an increased risk of type 1 DM.
Limitations
We have many limitations in the study. As in our study, case-control studies always have the potential for bias. It is not easy to collect accurate and unbiased data on past exposures. Therefore case-control studies are prone to some sources of bias like recall bias or the control group’s selection from the hospital. Many of the established risk factors were questioned, in order to overcome confounding. However, the gestation week was not questioned at birth, so it could not be evaluated whether the birth weight was normal for gestational age. It was questioned whether they drank water during exclusive breastfeeding, but we did not collect data on when they started to consume water. Therefore this variable maybe could provide a better comparison for exclusive breastfeeding duration in future studies. In addition, the vaccination status of the children was not asked and abortion was not researched while questioning the birth interval and birth order so they may be confounding factors. Since infections in the first three years were questioned by anamnesis, their bacterial / viral status could not be determined and their relationship with enteroviruses could not be investigated.
Conclusions
Longer exclusive breastfeeding duration may prevent the early introduction of certain nutrients in the diet. Determining the contribution of exclusive breastfeeding and its interactions with protective factors to the disease is important in establishing preventive policies. Breastfeeding is cost-effective and may be a free intervention for the prevention of type 1 DM. Support from partners is a key factor in maintaining breastfeeding in communities. Considering the limitations of the study, systematic reviews with meta-analysis are needed in determining the role of both exclusive and non-exclusive breastfeeding on the development of type 1 DM.
Availability of data and materials
The dataset could be obtained from the corresponding author upon reasonable request.
Abbreviations
- HLA:
-
Human leukocyte antigen
- PAR:
-
Population attributable risk
- PAR %:
-
Population attributable risk percent
References
National Diabetes Consensus Group. TURKDIAB Diabetes Diagnosis and Treatment Guideline 2017. 7th edition. İstanbul; 2017. http://www.turkdiab.org/admin/PICS/webfiles/Diyabet_tani_ve_tedavi__kitabi.pdf. Accessed 25 May 2021.
International diabetes federation. Diabetes atlas 2021. 10th ed. Brussels: International Diabetes Federation; 2021. https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_Edition_2021.pdf.
Turkey Endocrinology and Metabolism Society. Endocrinology and Metabolism Association Guideline 2019. 14th edition. Ankara; 2019. https://temd.org.tr/admin/uploads/tbl_kilavuz/20200625154506-2020tbl_kilavuz86bf012d90.pdf. Accessed 29 Apr 2020.
Åkerblom HK, Vaarala O, Hyöty H, Ilonen J, Knip M. Environmental factors in the etiology of type 1 diabetes. Am J Med Genet Semin Med Genet. 2002;115:18–29.
Esposito S, Toni G, Tascini G, Santi E, Berioli MG, Principi N. Environmental factors associated with type 1 diabetes. Front Endocrinol. 2019;10:592. https://doi.org/10.3389/fendo.2019.00592.
D’Angeli MA, Merzon E, Valbuena LF, Tirschwell D, Paris CA, Mueller BA. Environmental factors associated with childhood-onset type 1 diabetes mellitus: an exploration of the hygiene and overload hypotheses. Arch Pediatr Adolesc Med. 2010;164:732–8. https://doi.org/10.1001/archpediatrics.2010.115.
Redondo MJ, Concannon P. Genetics of type 1 diabetes comes of age. Diabetes Care. 2020;43:16–8. https://doi.org/10.2337/dci19-0049.
DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449–62. https://doi.org/10.1016/S0140-6736(18)31320-5.
Pociot F, Akolkar B, Concannon P, Erlich HA, Julier C, Morahan G, et al. Genetics of type 1 diabetes: What’s next? Diabetes. 2010;59:1561–71. https://doi.org/10.2337/db10-0076.
Howson JMM, Rosinger S, Smyth DJ, Boehm BO, Aldinger G, Aufschild J, et al. Genetic analysis of adult-onset autoimmune diabetes. Diabetes. 2011;60:2645–53. https://doi.org/10.2337/db11-0364.
Soltész G. Diabetes in the young: A paediatric and epidemiological perspective. Diabetologia. 2003;46:447–54. https://doi.org/10.1007/s00125-003-1101-0.
Piescik-Lech M, Chmielewska A, Shamir R, Szajewska H. Systematic review: early infant feeding and the risk of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2017;64:454–9.
Bodansky HJ, Staines A, Stephenson C, Haigh D, Cartwrigth R. Evidence for an environmental effect in the aetiology of insulin dependent diabetes in a transmigratory population. BMJ. 1992;304:1020–2. https://doi.org/10.1136/BMJ.304.6833.1020.
Muntoni S, Cocco P, Aru G, Cucca F, Muntoni S. Nutritional factors and worldwide incidence of childhood type 1 diabetes. Am J Clin Nutr. 2000;71:1525–9.
Aljabri KS, Bokhari SA, Khan MJ. Glycemic changes after vitamin D supplementation in patients with type 1 diabetes mellitus and vitamin D deficiency. Ann Saudi Med. 2010;30:454. https://doi.org/10.4103/0256-4947.72265.
Hyppönen E, Läärä E, Reunanen A, Järvelin MR, Virtanen SM. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet. 2001;358:1500–3.
Holmberg H, Whalberg J, Vaarala O, Ludvigsson J. Short duration of breast-feeding as a risk-factor for β-cell autoantibodies in 5-year-old children from the general population. Br J Nutr. 2007;97:111–6.
Knip M, Åkerblom HK, Altaji E, Becker D, Bruining J, Castano L, et al. Effect of hydrolyzed infant formula vs conventional formula on risk of type 1 diabetes the TRIGR randomized clinical trial. JAMA. 2018;319:38–48. https://doi.org/10.1001/jama.2017.19826.
Cardwell CR, Stene LC, Ludvigsson J, Rosenbauer J, Cinek O, Svensson J, et al. Breast-feeding and childhood-onset type 1 diabetes: A pooled analysis of individual participant data from 43 observational studies. Diabetes Care. 2012;35:2215–25. https://doi.org/10.2337/dc12-0438.
Xiao L, Van’t Land B, Engen PA, Naqib A, Green SJ, Nato A, et al. Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci Rep. 2018;8:1–15.
Virtanen SM, Knip M. Nutritional risk predictors of β cell autoimmunity and type 1 diabetes at a young age. Am J Clin Nutr. 2003;78:1053–67.
Esposito S, Toni G, Tascini G, Santi E, Berioli MG, Principi N. Environmental factors associated with type 1 diabetes. Front Endocrinol. 2019;10:592.
Norris JM, Barriga K, Klingensmith G, Hoffman M, Eisenbarth GS, Erlich HA, et al. Timing of initial cereal exposure in infancy and risk of islet autoimmunity. J Am Med Assoc. 2003;290:1713–20. https://doi.org/10.1001/jama.290.13.1713.
Virtanen SM, Takkinen HM, Nevalainen J, Kronberg-Kippilä C, Salmenhaara M, Uusitalo L, et al. Early introduction of root vegetables in infancy associated with advanced ß-cell autoimmunity in young children with human leukocyte antigen-conferred susceptibility to type 1 diabetes. Diabet Med. 2011;28:965–71. https://doi.org/10.1111/j.1464-5491.2011.03294.x.
Streisand R, Monaghan M. Young children with type 1 diabetes: challenges, research, and future directions. Curr Diabetes Rep. 2014;14:520.
World Health Organization. Classification of Diabetes Mellitus 2019. Geneva; 2019. https://www.who.int/publications/i/item/classification-of-diabetes-mellitus. Accessed 11 Apr 2020.
Social Security Institution of Turkey. Diabetes from the Social Security Institution’s Perspective. Ankara; 2014. https://veri.sgk.gov.tr. Accessed 11 Apr 2020.
World Health Organization; International Diabetes Federation. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia Report of a WHO/IDF Consultation. Geneva; 2006. https://apps.who.int/iris/bitstream/handle/10665/43588/9241594934_eng.pdf?sequence=1&isAllowed=y. Accessed 1 Jan 2021.
Awadalla NJ, Hegazy AA, El-Salam MA, Elhady M. Environmental factors associated with type 1 diabetes development: A case control study in Egypt. Int J Environ Res Public Health. 2017;14:615. https://doi.org/10.3390/ijerph14060615.
Muntoni S, Mereu R, Atzori L, Mereu A, Galassi S, Corda S, et al. High meat consumption is associated with type 1 diabetes mellitus in a Sardinian case-control study. Acta Diabetol. 2013;50:713–9.
Knip M, Simell O. Environmental triggers of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2:1–15.
Knip M, Virtanen SM, Becker D, Dupre J, Krischer JP, A HK. Early feeding and risk of type 1 diabetes: experiences from the trial to reduce insulin-dependent diabetes mellitus in the genetically at risk (TRIGR) 1-6. Am J Clin Nutr. 2011;94:1814S–20.
Nygren M, Carstensen J, Koch F, Ludvigsson J, Frostell A. Experience of a serious life event increases the risk for childhood type 1 diabetes : the ABIS population-based prospective cohort study. Diabetologia. 2015;58:1188–97.
Rewers M, Ludvigsson J. Environmental risk factors for type 1 diabetes. Lancet. 2016;387:2340–8. https://doi.org/10.1016/S0140-6736(16)30507-4.
World Health Organization. Exclusive breastfeeding for optimal growth, development and health of infants. 2019. https://www.who.int/elena/titles/exclusive_breastfeeding/en/. Accessed 2 Jan 2021.
Neyzi O, Günöz H, Furman A, Bundak R, Gökçay G, Darendeliler F, et al. Body weight, height, head circumference and body mass index reference values in Turkish children. Cocuk Sagligi ve Hastalikari Dergisi. 2008;51(1):1.
World Health Organization. Health Topics: Obesity. https://www.who.int/health-topics/obesity#tab=tab_1. Accessed 2 Jan 2021.
Republic of Turkey Ministry of Health, General Directorate of Public Health. Turkey Physical Activity Guidelines. 2nd edition. Ankara; 2014. https://hsgm.saglik.gov.tr/depo/birimler/saglikli-beslenme-hareketli-hayat-db/Fiziksel_Aktivite_Rehberi/Turkiye_Fiziksel_Aktivite_Rehberi.pdf.
Hennekens CH, Buring JE. Measures of Disease Frequency and Association. In: Mayrent SL, editor. Epidemiology in Medicine. 1st ed. Boston: Little Brown; 2012. p. 96.
Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. JAMA. 2003;290:1721–8. https://doi.org/10.1001/JAMA.290.13.1721.
Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL. Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr. 1995;21:383–6. https://doi.org/10.1097/00005176-199511000-00003.
World Health Organization. Health Topics: Breastfeeding. https://www.who.int/health-topics/breastfeeding#tab=tab_1. Accessed 25 Jan 2022.
Hacettepe University Institute of Population Studies. Turkey Demographic and Health Survey 2018 Main Report. Ankara/Turkey; 2019. www.hips.hacettepe.edu.tr. Accessed 9 Nov 2021.
Centers for Disease Control and Prevention. Results: Breastfeeding Rates. https://www.cdc.gov/breastfeeding/data/nis_data/results.html. Accessed 11 Nov 2021.
Çarkçı NŞ, Altuğ ÖS. Studying the epidemiologic characteristics of children with type 1 diabetes followed in İzmir. J Educ Res Nurs. 2020;17:24–31.
Ziegler AG, Schmid S, Huber D, Hummel M, Bonifacio E. Early infant feeding and risk of developing type 1 diabetes-associated autoantibodies. J Am Med Assoc. 2003;290:1721–8. https://doi.org/10.1001/jama.290.13.1721.
Virtanen SM, Kenward MG, Erkkola M, Kautiainen S, Kronberg-Kippilä C, Hakulinen T, et al. Age at introduction of new foods and advanced beta cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes. Diabetologia. 2006;49:1512–21. https://doi.org/10.1007/s00125-006-0236-1.
Viner RM, Hindmarsh PC, Taylor B, Cole TJ. Childhood body mass index (BMI), breastfeeding and risk of type 1 diabetes: findings from a longitudinal national birth cohort. Diabet Med. 2008;25:1056–61. https://doi.org/10.1111/J.1464-5491.2008.02525.X.
Frederiksen B, Kroehl M, Lamb MM, Seifert J, Barriga K, Eisenbarth GS, et al. Infant exposures and development of type 1 diabetes mellitus: the diabetes autoimmunity study in the young (DAISY). JAMA Pediatr. 2013;167:808–15. https://doi.org/10.1001/JAMAPEDIATRICS.2013.317.
Lund-Blix NA, Sander SD, Størdal K, Nybo Andersen AM, Rønningen KS, Joner G, et al. Infant feeding and risk of type 1 diabetes in two large Scandinavian birth cohorts. Diabetes Care. 2017;40:920–7. https://doi.org/10.2337/DC17-0016/-/DC1.
Ponsonby AL, Pezic A, Cochrane J, Cameron FJ, Pascoe M, Kemp A, et al. Infant anthropometry, early life infection, and subsequent risk of type 1 diabetes mellitus: a prospective birth cohort study. Pediatr Diabetes. 2011;12:313–21. https://doi.org/10.1111/J.1399-5448.2010.00693.X.
Savilahti E, Saarinen KM. Early infant feeding and type 1 diabetes. Eur J Nutr. 2009;48:243–9. https://doi.org/10.1007/S00394-009-0008-Z.
UNICEF. Breastfeeding: A Mother’s Gift, for Every Child. New York; 2018. https://www.unicef.org/media/48046/file/UNICEF_Breastfeeding_A_Mothers_Gift_for_Every_Child.pdf. Accessed 2 Feb 2022.
Tohotoa J, Maycock B, Hauck YL, Howat P, Burns S, Binns CW. Dads make a difference: an exploratory study of paternal support for breastfeeding in Perth, Western Australia. Int Breastfeed J. 2009;4:15. https://doi.org/10.1186/1746-4358-4-15.
Šipetić S, Vlajinac H, Kocev N, Bjekić M, Sajic S. Early infant diet and risk of type 1 diabetes mellitus in Belgrade children. Nutrition. 2005;21:474–9. https://doi.org/10.1016/J.NUT.2004.07.014.
Knip M, Virtanen SM, Åkerblom HK. Infant feeding and the risk of type 1 diabetes. Am J Clin Nutr. 2010;91:1506S. https://doi.org/10.3945/AJCN.2010.28701C.
Virtanen SM, Nevalainen J, Kronberg-Kippilä C, Ahonen S, Tapanainen H, Uusitalo L, et al. Food consumption and advanced β cell autoimmunity in young children with HLA-conferred susceptibility to type 1 diabetes: a nested case-control design. Am J Clin Nutr. 2012;95:471–8. https://doi.org/10.3945/AJCN.111.018879.
Wahlberg J, Vaarala O, Ludvigsson J. Dietary risk factors for the emergence of type 1 diabetes-related autoantibodies in 21/2 year-old Swedish children. Br J Nutr. 2006;95:603–8. https://doi.org/10.1079/BJN20051676.
Patterson C. Rapid early growth is associated with increased risk of childhood type 1 diabetes in various European populations. Diabetes Care. 2002;25:1755–60. https://doi.org/10.2337/DIACARE.25.10.1755.
Barbeau WE, Bassaganya-Riera J, Hontecillas R. Putting the pieces of the puzzle together - a series of hypotheses on the etiology and pathogenesis of type 1 diabetes. Med Hypotheses. 2007;68:607–19. https://doi.org/10.1016/J.MEHY.2006.07.052.
Maurano F, Mazzarella G, Luongo D, Stefanile R, D’Arienzo R, Rossi M, et al. Small intestinal enteropathy in non-obese diabetic mice fed a diet containing wheat. Diabetologia. 2005;48:931–7. https://doi.org/10.1007/S00125-005-1718-2.
Lund-Blix NA, Dong F, Mårild K, Seifert J, Barón AE, Waugh KC, et al. Gluten intake and risk of islet autoimmunity and progression to type 1 diabetes in children at increased risk of the disease: the diabetes autoimmunity study in the young (DAISY). Diabetes Care. 2019;42:789. https://doi.org/10.2337/DC18-2315.
Haupt-Jorgensen M, Holm LJ, Josefsen K, Buschard K. Possible prevention of diabetes with a gluten-free diet. Nutrients. 2018;10. https://doi.org/10.3390/NU10111746.
Republic of Turkey Ministry of Food Agriculture and Livestock. Republic of Turkey Ministry of Food Agriculture and Livestock GAP International Agricultural Research and Training Center for Grain Report. Diyarbakır; 2013. https://arastirma.tarimorman.gov.tr/gaputaem/Belgeler/tarımsalveriler/gaputaemgncel/TahılRaporu.pdf. Accessed 29 Jan 2022.
Acknowledgments
The authors are grateful for help and support provided by Prof. Dr. Damla Gökşen Şimşek, Assoc. Prof. Dr. Aslı Aslan, Spec. Dr. Eren Er and all health professionals from Ege University who enabled contact with potential cases and controls during the data collection.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
R.D., and İ.Ç. contributed to the design and implementation of the research and to the analysis of the results. İ.Ç. contributed to the writing of the manuscript. R.D. encouraged İ.Ç. to investigate and she supervised this work. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ege University Faculty of Medicine Clinical Research Ethics Committee (decision no.10059, dated 9 January 2020). Written approval was obtained from the administration of the Department of Child Health and Diseases before starting the study.
Consent for publication
Not applicable.
Competing interests
We have no known conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Çiçekli, İ., Durusoy, R. Breastfeeding, nutrition and type 1 diabetes: a case-control study in Izmir, Turkey. Int Breastfeed J 17, 42 (2022). https://doi.org/10.1186/s13006-022-00470-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13006-022-00470-z